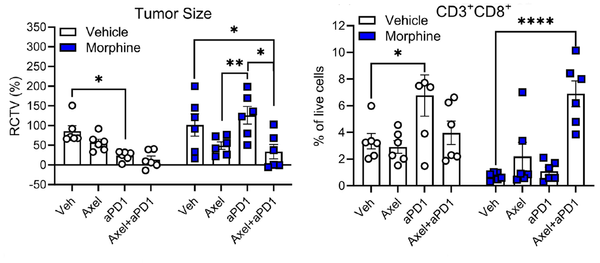
San Francisco, CA – December 04, 2024 – Glycyx, a clinical-stage biopharmaceutical company advancing axelopran, a novel solution to improve outcomes for cancer patients undergoing immunotherapy, today spotlighted a significant new publication in the Journal for ImmunoTherapy of Cancer (JITC), that underscores the urgent need to address opioid-induced immunotherapy failure (OIIF).
The study, led by Nicole N. Scheff, Ph.D., assistant professor in the Department of Neurobiology at the University of Pittsburgh and UPMC Hillman Cancer Center, provides critical insights into the mechanisms by which opioids impair immune checkpoint inhibitor (ICI) efficacy. The findings are a collaborative effort with clinical input from Dan P. Zandberg, M.D., associate professor of medicine at Pitt and co-leader of the UPMC Hillman Head and Neck Program and Marci Nilsen, Ph.D., professor in the Department of Otolaryngology-Head and Neck Surgery at Pitt and director of the Head and Neck Survivorship Clinic at UPMC Hillman. This translational team highlights how opioids contribute to immunosuppression and tumor progression, reinforcing the foundational science behind Glycyx’s lead candidate, axelopran.
“Dr. Scheff’s work represents a groundbreaking contribution to understanding the interaction between opioid use and immune suppression in cancer treatment. Axelopran is designed to block the negative effects of opioids on cancer treatment while preserving their pain-relieving properties,” said Justin Chickles, CEO of Glycyx. “This independent research aligns with Glycyx’s mission to address this critical barrier to effective immunotherapy and strengthen the scientific foundation for our development of axelopran.”
Key Milestones Achieved by Glycyx
- Active IND Status: Axelopran is now officially under an Investigational New Drug (IND) application with the FDA, paving the way for clinical trials.
- Clinical Supply Produced: Glycyx has successfully completed the production of clinical-grade axelopran, ensuring readiness for trial initiation.
- Capital Raise in Progress: The company is actively raising funds for its upcoming human clinical trials, leveraging the strong preclinical and regulatory foundation to drive its innovative treatment forward.